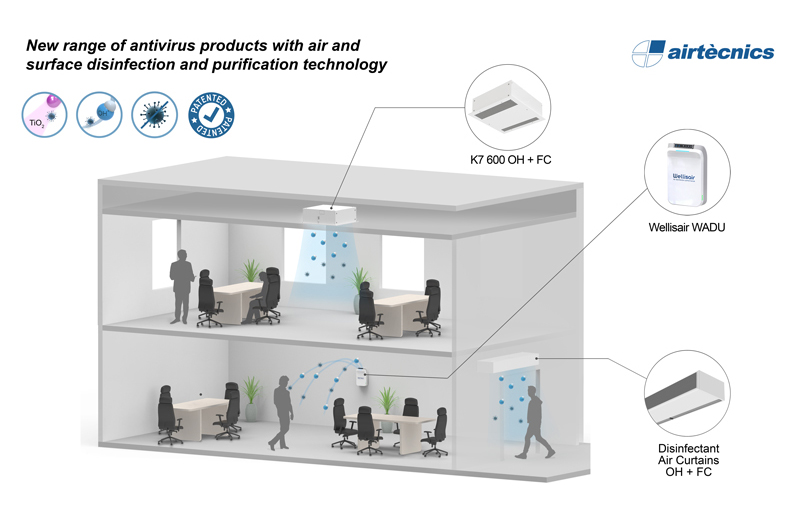
Airtècnics realeses the hydrogen peroxide cartridge compatible with Wellisair purification unit
Airtècnics developed two types of cartridges compatible with the Wellisair device, with either D-limonene or hydrogen peroxide (H2O2). Both of them have been tested for safety and efficacy against viruses, bacteria and air quality (VOCs, particles and odors).

Thanks to the innovative, patented technology based on Advanced Oxidation Processes (AOP), Wellisair uses hydroxyl radicals to inactivate 99.9% of microorganisms, eliminate odors and reduce Volatile Organic Compounds (VOCs) and PM particles in suspension.
Hidroxyl radicals clean the atmosphere
The hydroxyl radical is a molecule consisting of a hydrogen atom and an oxygen atom with a free electron (unpaired). is the most unstable species within the Reactive Oxygen Species (ROS), a group of unstable molecules that oxidize others to return to equilibrium, getting them destabilized and generating reaction cascades. If the threshold tolerated by cells is overstepped, oxidative stress given by the damaging effects of hydroxyl radicals leads to cell death.
Hydroxyl radicals are the product of a great amount of intercommunicated reactions. They are mainly formed outdoors by the photolysis of ozone, while the principal source indoors is the ozonolysis of alkenes.
Alkenes in general produce hydroxyl radicals, but D-limonene stands out among them in being the alkene with the greatest capacity to generate hydroxyl radicals indoors when reacting with ozone3. D-limonene is a natural substance, structurally a terpene, derived from citrus fruits.

The monitored amount of ozone emitted by Wellisair (<0.02 ppm) is enough to provoke the first radicals to contact D-limonene, which because of their high instability will react, giving rise to other species such as oxygen (O2), water (H2O), or carbon dioxide (CO2). In addition, comply with the limits adopted by the National Institute of Safety and Health (INSS). These parameters include ozone emissions (<0,05ppm) and hydrogen peroxide emissions (<1ppm or <0,1mg/m3). Wellisair has been tested in independent laboratories and more than complies with both limits.
The “peroxone” process is an AOP based on ozone decomposition (in this case, emitted at low concentrations by the device) after making contact with hydrogen peroxide (coming out from the cartridge), resulting in molecules of water (H2O), oxygen (O2) and hydroxyl radicals, mainly. Additionally, hydroxyl radicals are generated when sunlight at wavelengths less than 360 nm falls upon hydrogen peroxide, and therefore incident light through the window favors an oxidative environment.